Our Team
We are scientists, drug developers, clinicians, pathologists, bioinformaticians, engineers, innovators, and cancer warriors redefining the treatment landscape for patients with recalcitrant cancers.
Management
 Joseph Paul Eder, MD
Joseph Paul Eder, MD
Chief Medical Officer
Dr. Eder is Chief Medical Officer at Incendia, bringing over 30 years of clinical experience at leading cancer research institutions as well as the pharmaceutical industry. Most recently, Dr. Eder was Clinical Director of the Early Drug Development Program as well as Professor at the Yale School of Medicine. From 2010 to 2012, Dr. Eder was Medical Science Director, Disease Area Clinical Expert, Hematology, AstraZeneca, PLC as well as Associate Clinical Professor, Harvard Medical School, Boston, MA. Dr. Eder was Clinical Discovery Sr. Director, Oncology, AstraZeneca, PLC from 2009 to 2012, prior to which he was Medical Science Director at AstraZeneca. From 2005 to 2010, Dr. Eder was Associate Professor, Harvard Medical School and from 2003 to 2007 was Clinical Director, Clinical Research Center at the Dana-Farber Cancer Institute. He was Clinical Director, Experimental Therapeutics & Translational Pharmacology, Dana-Farber Harvard Cancer Center from 1998 to 2007, and was Assistant Professor, Harvard Medical School from 1988 to 2004. From 1985 to 1988 Dr. Eder was Instructor, Harvard Medical School. Dr. Eder was awarded his MD from Georgetown University School of Medicine.
 John M. Goldberg, MD
John M. Goldberg, MD
Acting Chief Development Officer
Dr. Goldberg is Acting Chief Development Officer at Incendia, responsible for driving the company’s human trials to proof of concept, and for communicating our clinical progress and vision to external stakeholders. He brings 21 years of experience in academic and industry drug development to Incendia, and his most recent roles include Chief Medical Officer at Rafael Holdings, a family office where he advised the principal on new asset investment, and Chief Medical Officer at Oncorus, a venture backed immunotherapy company where he led clinical and helped with significant fundraising efforts. Before Oncorus, he held positions in industry at H3 Biomedicine and Agenus. He led the pediatric oncology Phase 1 program at the University of Miami from 2008-2015.
Dr. Goldberg trained in pediatric hematology-oncology at Dana-Farber/Boston Children’s Hospital and in general pediatrics at the University of Rochester. He holds an MD from the University of Massachusetts Chan Medical School, an AB in Biological Sciences from the University of Chicago and serves on the Medical Advisory Board of the Sarcoma Foundation of America.
 Brian Kiraly
Brian Kiraly
Acting Head of Finance
Brian brings 25 years of financial leadership and hands-on experience in biotech from pre-IPO through product commercialization including strategy, long-range planning, investment planning, accounting, and SEC reporting.
Prior to joining the Incendia team in February 2026, Brian was Senior Vice President of Finance and Accounting at Sage Therapeutics where he led Long Range Planning as well as operational finance functions. At Sage, Brian was involved in the IPO, over $2B in equity financings, $1.6B through therapeutic partnerships with Biogen and Shionogi, and the acquisition of Sage by Supernus in 2025.
Previously, Brian held financial leadership positions at Vertex Pharmaceuticals and Millennium Pharmaceuticals. He began his career in Management Consulting with Arthur D. Little and Consumer Products with Procter and Gamble. Brian received his undergraduate degree in Electrical Engineering at The University of Texas and his MBA at Northwestern University.
 Susan G. Macdonald, PhD
Susan G. Macdonald, PhD
SVP, Non Clinical Development & Strategy
Sue is Senior Vice President of Non Clinical Development & Strategy at Incendia. She is an experienced biotech executive with more than 25 years of experience in research and development, spanning several therapeutic areas and therapeutic modalities. Her expertise includes basic and translational research, leading cross-functional discovery and development programs, developing strategies for projects and portfolios, and alliance management. She has deep experience in preparing and submitting INDs/CTAs, including meeting with regulatory agencies in North America and Europe, and in the development of strategies for programs through registration. She most recently served as Vice President of Research and Development at Aldeyra Therapeutics. Prior to joining Aldeyra, she held senior roles in R&D Strategy, Portfolio, and Project Management at Alexion Pharmaceuticals and Program Management at NKT Therapeutics. She has also served in a variety of roles at Archemix, ArQule, TEI Biosciences, and Onyx Pharmaceuticals, where, as a founding employee, she conducted research that enabled the discovery of sorafenib (Nexavar®). Sue also advises and helps emerging companies navigate the intersecting paths of strategy, science, and development.
Sue received her undergraduate degree in biology from Hobart and William Smith Colleges and her Ph.D. in Physiology from the University of Massachusetts Medical School. She completed her postdoctoral training at Onyx Pharmaceuticals, during which she received a prestigious NIH National Research Service Award.
 Wendye R. Robbins, MD
Wendye R. Robbins, MD
President & Chief Executive Officer
Wendye has been our President and CEO since June 2023. She has made a career of partnering with investors to build companies from the ground up. She has raised over $1B across her companies in public and private markets. She has been involved in the development of multiple small and large molecule therapeutics including Qutenza™, Ajovy™ and others in the development pipeline. Among her previous engagements, she collaborated with investors to incubate or build multiple nascent biotechs including NeurogesX, Inc. (NASD: NGSX, sold to Acorda Therapeutics), Labrys Biologics (sold to TEVA 2014), Decibel Therapeutics (sold to Regeneron 2023), and Blade Therapeutics. In 2025, she joined the BIO Board of Directors, Emerging Companies Division. From 2019 – 2025, she served as director of RAPT Therapeutics (NASDAQ: RAPT) and chaired RAPT’s Pricing and Transaction subcommittees. Wendye is a frequent mentor to entrepreneurs, graduate students and house staff. From 2004 - 2016, Wendye was privileged to serve as Clinical Associate Professor in the Department of Anesthesiology at Stanford University School of Medicine. Previously, she served as Assistant Professor at the University of California, San Francisco. Wendye completed post graduate training in anesthesiology and pain medicine at Johns Hopkins University School of Medicine, Department of Anesthesia and Critical Care Medicine. She interned in internal medicine at the Hospital of the University of Pennsylvania. She obtained her MD from the Medical College of Pennsylvania and her BS from the University of California, Haas School of Business. She is board certified in anesthesiology.
 Thomas Schürpf, PhD
Thomas Schürpf, PhD
SVP, Research
Before joining Incendia Therapeutics, Thomas was a Director of Discovery Biology and first scientist hired at Scholar Rock, Inc., a Cambridge biotechnology company that focuses on the discovery and development of novel growth factor-targeting antibodies for the treatment of musculoskeletal diseases, fibrosis, and cancer. During his nine year tenure at Scholar Rock, Thomas built up the company’s TGFβ portfolio and led the discovery of SRK-181, a selective inhibitor of latent TGFβ1 activation that is currently being investigated in the DRAGON Phase 1 clinical trial in patients with locally advanced or metastatic solid tumors. He is an inventor on several granted patents and international patent applications. Thomas received postgraduate training at Harvard Medical School and Children’s Hospital Boston in the laboratory of Dr. Timothy Springer and received a Ph.D from ETH Zurich, Switzerland.
 Irena Lerentracht Webster, MPH
Irena Lerentracht Webster, MPH
SVP, Development and Operations
Irena Webster is a seasoned global drug development leader and board member with over 20 years of industry, CRO, and site-level experience. Irena is passionate about innovative trial execution with a focus on the patient and caregiver journey. She also participates in forums and initiatives focused on DE&I in trial awareness and access as well as career development and exposure across STEM.
Before joining Incendia, Irena served as Vice President of Program Strategy & Development Operations for Arkuda Therapeutics, a biotechnology company focused on rare neurodegenerative disease. Prior to that, as Vice President of Development Operations, she led the Clinical Operations and Biometrics teams at FORMA Therapeutics (now a Novo Nordisk company) through a global sickle cell program as well as the approval of olutasidanib (Rezlidhia™ now a Rigel product) for the treatment of IDH1 mutation Acute Myeloid Leukemia.
Prior to Forma, Irena served as Director, Head of Early Clinical Development Operations at Sage Therapeutics, where she led an operational team that worked closely with the lead optimization, translational, and medical teams focused on experimental medicine, including signal-finding trials and biomarker development in neurological disorders and rare disease. Prior to Sage, she worked at Alkermes where she was the clinical operations lead on multiple programs across depression, schizophrenia, addiction and reward disorders. Irena began her career at Kos Pharmaceuticals, a pharma company focused on cardiovascular drug development, which was later acquired by Abbot. Throughout her career, she has contributed to multiple NDAs and sNDAs. Irena earned a Master of Public Health degree from New York Medical College.
Facilities Management
 Sumita Roy, MS, CG(ASCP)CM
Sumita Roy, MS, CG(ASCP)CM
Senior Director, Operations, Procurement, Lab & Facilities Operations
Prior to joining Incendia Therapeutics Inc., Sumita was the Director of Internal Operations at Sotio Biotech, Inc., a biotechnology company focused on innovative cancer immunotherapies. Sumita has over 25 years of experience in lab and facilities operations with focus on quality, efficiency, and cost reduction. She has developed purchasing strategies, procurement plans, has spearheaded implementation and roll out of ERP systems, and overseen lab safety, compliance and asset management. She has extensive experience in laboratory accreditation and in GLP, CLIA and Sarbanes Oxley policies. She supervised the buildout of GMP facility at Sotio Biotech, Inc. and a state-of-the-art 60,000 sf laboratory and office space at Editas Medicine. As Director of Operations at Brigham & Women’s Hospital (Pathology), Harvard Medical School, Sumita led the setup of a biorepository.
Sumita received her undergraduate degree in Biology and her M.S. degree in Cytogenetics from the University of Kalyani, India. She received her postgraduate training in genetics and prenatal diagnostics at The Children’s Hospital Boston, Harvard Medical School. Sumita’s expertise in procurement management using Chemdex was acclaimed in Fortune, a global business magazine.
Board of Directors
 Michael Kauffman, MD, PhD
Michael Kauffman, MD, PhD
Chairman of the Board, Incendia Therapeutics, CEO Nereid Therapeutics
Michael Kauffman MD PhD has spent the last 28 years in the pharmaceutical industry. He played major leadership roles in the development and approval of three cancer drugs: Velcade®, Kyprolis®, and Xpovio®. He is the lead director or chairman of the board for Incendia Therapeutics, Verastem Oncology, and BiVictriX Therapeutics, and a board member at Kezar Life Sciences and Adicet Bio. Dr. Kauffman was co-founder and previously served as Chief Executive Officer of Karyopharm, where he guided the company’s transition from a discovery-stage biotechnology company to a commercial-stage organization with the global approvals of XPOVIO®, and served as Senior Medical Advisor thereafter. Dr. Kauffman was also the previous Chief Medical Officer of Onyx Pharmaceuticals Inc. where he lead the development and approval of Kyprolis®. Prior to that he served as President and Chief Executive Officer of EPIX Pharmaceuticals, Inc. (previously Predix Pharmaceuticals, Inc.), and was Vice President of Clinical Development and Program Lead for Velcade®. Dr. Kauffman received his M.D. and Ph.D. from Johns Hopkins Medical School, trained at Beth Israel and Massachusetts General Hospitals in Boston, and is board certified in Internal Medicine.
 Sakae Asanuma
Sakae Asanuma
President Taiho Ventures
Sakae Asanuma established Taiho Ventures as the founding President in 2016. Prior to joining Taiho, he was President and CEO at Astellas Venture Management and US Head of Astellas Innovation Management in 2011-2015. Prior to Astellas, he worked for Yasuda Enterprise, a Japan/US-based VC firm in 2000-2011. He has invested in several dozen of biotech companies since 2000 and majority of his portfolios achieved IPOs or M&AS. During his tenure at Taiho and Astellas, he led to executed dozens of research collaboration deals with academia and biotech ventures, including several build-to-buys or spin-outs.
 Louis G. Lange, MD, PhD
Louis G. Lange, MD, PhD
General Partner
Asset Management Ventures, Inc.
Louis G. Lange is a highly successful biotechnology investor and serial entrepreneur with deep experience building companies that have generated scientific breakthroughs and significant value for patients and investors. Since 2009, Dr. Lange has served as General Partner with Asset Management Ventures (AMV), Inc., a venture capital firm investing in early-stage digital health, technology and life sciences companies. Dr. Lange focuses on healthcare innovation, investing in more than 50 companies.
Dr. Lange has 22 years of experience in academic medicine at Harvard and Washington University, where he served as Chief of Cardiology and Professor of Medicine at Jewish Hospital, where he was one of the first academicians in molecular cardiology. Based on experience within this field, Dr. Lange founded CV Therapeutics in 1992, leading the company’s IPO in 1996 and research, development and launches of Ranexa®, a first-in-class late sodium channel blocker, and Lexiscan®, a first-in-class adenosine A2a receptor agonist. Dr. Lange oversaw the commercial success of CV Therapeutics and its sale to Gilead in 2009 at an 85 percent premium. He provided strategic counsel as a member of the Gilead Operating Committee over the next 10 years, during which the company’s market capitalization nearly tripled.
As an entrepreneur, Dr. Lange has also founded RPS (2010), Cardiogen Sciences (2014), Amygdala Neurosciences, Inc. (2015) and Recardia Therapeutics (2018). RPS and Cardiogen were profitably sold to GE and Audentes Therapeutics, respectively, in 2015 and 2016. He remained as Lead Director of Audentes until its sale to Astellas for $3 billion in 2020.
Throughout his career, Dr. Lange has served on numerous public and private Boards in both the non-profit and for-profit arena. In addition to Incendia, he currently serves as a board member with NewAmsterdam Pharma Company (Nasdaq: NAMS).
He earned a bachelor’s degree from the University of Rochester, and an M.D. and a Ph.D. in Biological Chemistry from Harvard University.
 Steve Liapis
Steve Liapis
Managing Director Northpond Ventures
Steve Liapis is a Managing Director at Northpond Ventures where he focuses on biotechnology platforms and therapeutics investments. He also leads Northpond’s newco incubation efforts with the Wyss Institute at Harvard and the School of Engineering at MIT. Steve is Board Director at Walking Fish Therapeutics, Kyverna Therapeutics, Garuda Therapeutics, Opna Bio, Incendia Therapeutics, and Totus Medicines. Previously, Steve was Director of Oncology Portfolio Strategy at Sanofi, where he led global strategy and resource prioritization for Sanofi Oncology. Prior to Sanofi, Steve was Head of Strategy at Arbor Biotechnologies and served in leadership positions at L.E.K. Consulting where he focused on R&D and commercial strategy for immuno-oncology as well as advanced therapeutic modalities including gene therapy, gene editing, and cell therapy. Steve holds a Ph.D. in molecular biology from Harvard University where he trained in the laboratory of Dr. John Rinn, focusing on the discovery and molecular characterization of novel long noncoding RNAs (lncRNAs), as well as identifying the role of lncRNAs in disease pathogenesis.
 Christopher O’Donnell, Ph.D.
Christopher O’Donnell, Ph.D.
Partner Pfizer Ventures
Christopher O’Donnell, Ph.D. is Vice President, WRDM and Partner at Pfizer Ventures. Chris is responsible for identifying, evaluating, making and managing equity investments aligned with the future directions of Pfizer. He currently has responsibility for Pfizer’s investments in Adapsyn Bioscience, Arkuda Therapeutics, Arrakis Therapeutics, Kestrel Therapeutics, Mitokinin, MindImmune, Incendia Therapeutics, Pyxis Oncology (PYXS), Storm Therapeutics, Strata Oncology and Triana Biomedicine. He is also responsible for managing our investment in the Mission BioCapital and Phoenix Venture Partners funds. His prior investment responsibilities include BioAtla (BCAB), Kymera Therapeutics (KYMR), Morphic Therapeutic (MORF) and Petra Pharma.
Chris brings 20+ years of scientific leadership, a strong track record of delivering clinical candidates across multiple disease areas and modalities, and proven ability to build and lead highly engaged teams. He built and led the Applied Synthesis Technologies group within R&D to help accelerate the delivery of Pfizer’s small molecule portfolio. Prior to that, Chris built and led Pfizer’s Antibody Drug Conjugate Oncology Medicinal Chemistry group which delivered new linker, payload and conjugation methods resulting in over 7 conjugates entering clinical development. Chris started his career in the Neuroscience Medicinal Chemistry group where he invented and helped deliver numerous clinical candidates.
Chris received his BS in Chemistry from the University of Illinois-Urbana/Champaign and his PhD in Chemistry from the University of Wisconsin-Madison and joined Pfizer after completing post-doctoral studies at the University of California – Irvine.
 Wendye Robbins, MD
Wendye Robbins, MD
President & CEO
Wendye has been our President and CEO since June 2023. She has made a career of partnering with investors to build companies from the ground up. She has raised over $1B across her companies in public and private markets. She has been involved in the development of multiple small and large molecule therapeutics including Qutenza™, Ajovy™ and others in the development pipeline. Among her previous engagements, she collaborated with investors to incubate or build multiple nascent biotechs including NeurogesX, Inc. (NASD: NGSX, sold to Acorda Therapeutics), Labrys Biologics (sold to TEVA 2014), Decibel Therapeutics (sold to Regeneron 2023), and Blade Therapeutics. In 2025, she joined the BIO Board of Directors, Emerging Companies Division. From 2019 – 2025, she served as director of RAPT Therapeutics (NASDAQ: RAPT) and chaired RAPT’s Pricing and Transaction subcommittees. Wendye is a frequent mentor to entrepreneurs, graduate students and house staff. From 2004 - 2016, Wendye was privileged to serve as Clinical Associate Professor in the Department of Anesthesiology at Stanford University School of Medicine. Previously, she served as Assistant Professor at the University of California, San Francisco. Wendye completed post graduate training in anesthesiology and pain medicine at Johns Hopkins University School of Medicine, Department of Anesthesia and Critical Care Medicine. She interned in internal medicine at the Hospital of the University of Pennsylvania. She obtained her MD from the Medical College of Pennsylvania and her BS from the University of California, Haas School of Business. She is board certified in anesthesiology.
Company Advisors
GI Oncologist, Early Drug Development Program, Yale
Former Head of the IO Center Cordeliers, Paris
Dean for Collaborative Research and Partnerships
Director, Institute for Liver Research
Icahn School of Medicine at Mount Sinai
Institut Bergonie, Early Phase Trials and Sarcoma Units
Director, Early Phase Clinical Trials Unit UCSF
Dept. of Biomed Engineering, University of Minnesota
Former CMO & CDO Oncology Pfizer
Retired Chief Financial Officer Incendia Therapeutics
Michael Cecchini, MD
GI Oncologist, Early Drug Development Program, Yale
Assistant Professor of Medicine (Medical Oncology) Yale University School of Medicine
Hervé Fridman, MD PhD
Former Head of the IO Center Cordeliers, Paris
Professor Emeritus University of Paris School of Medicine Leader of Cancer Immunotherapy Group Cordeliers Research Center
Scott L. Friedman, MD
Dean for Collaborative Research and Partnerships
Director, Institute for Liver Research
Icahn School of Medicine at Mount Sinai
Antoine Italiano, MD, PhD
Institut Bergonie, Early Phase Trials and Sarcoma Units
Professor and Head of the Early Phase Trials and Sarcoma Units at Institut Bergonié, Bordeaux, France
Pamela Munster, MD
Director, Early Phase Clinical Trials Unit UCSF
Professor, Department of Medicine (Hematology/Oncology) University of California San Francisco Early Phase Clinical Trials Unit Co-leader of the Center for BRCA Research Co-leader of the Molecular Oncology Program
Paolo Provenzano, PhD
Dept. of Biomed Engineering, University of Minnesota
Associate Professor, Department of Biomedical Engineering University of Minnesota
Mace Rothenberg, MD
Former CMO & CDO Oncology Pfizer
Former Chief Medical Officer Pfizer Former Professor of Medicine and Ingram Professor of Cancer Research at Vanderbilt Independent Consultant
Bradford Smith
Retired Chief Financial Officer
Former Chief Financial Officer Incendia Therapeutics
Our Science
The TME is a complex ecosystem of cells and non-cellular components that both surrounds and penetrates within solid tumors. The TME influences tumor development, tumor immunity, metastasis, and response to anti-tumor therapies.
In solid cancers, the TME presents structural and biochemical barriers that can inhibit immune cell access to tumor cells, block immune cell activation, and diminish the effectiveness of treatment, including with immunotherapies. Response rates, including progression-free survival, remain very low across many treatments for solid cancers, particularly among advanced patients, in cancers such as thymic, pancreatic, ovarian, colorectal, lung and breast.
Incendia believes that there are multiple novel opportunities within the TME to target treatment of cancer and improve outcomes.
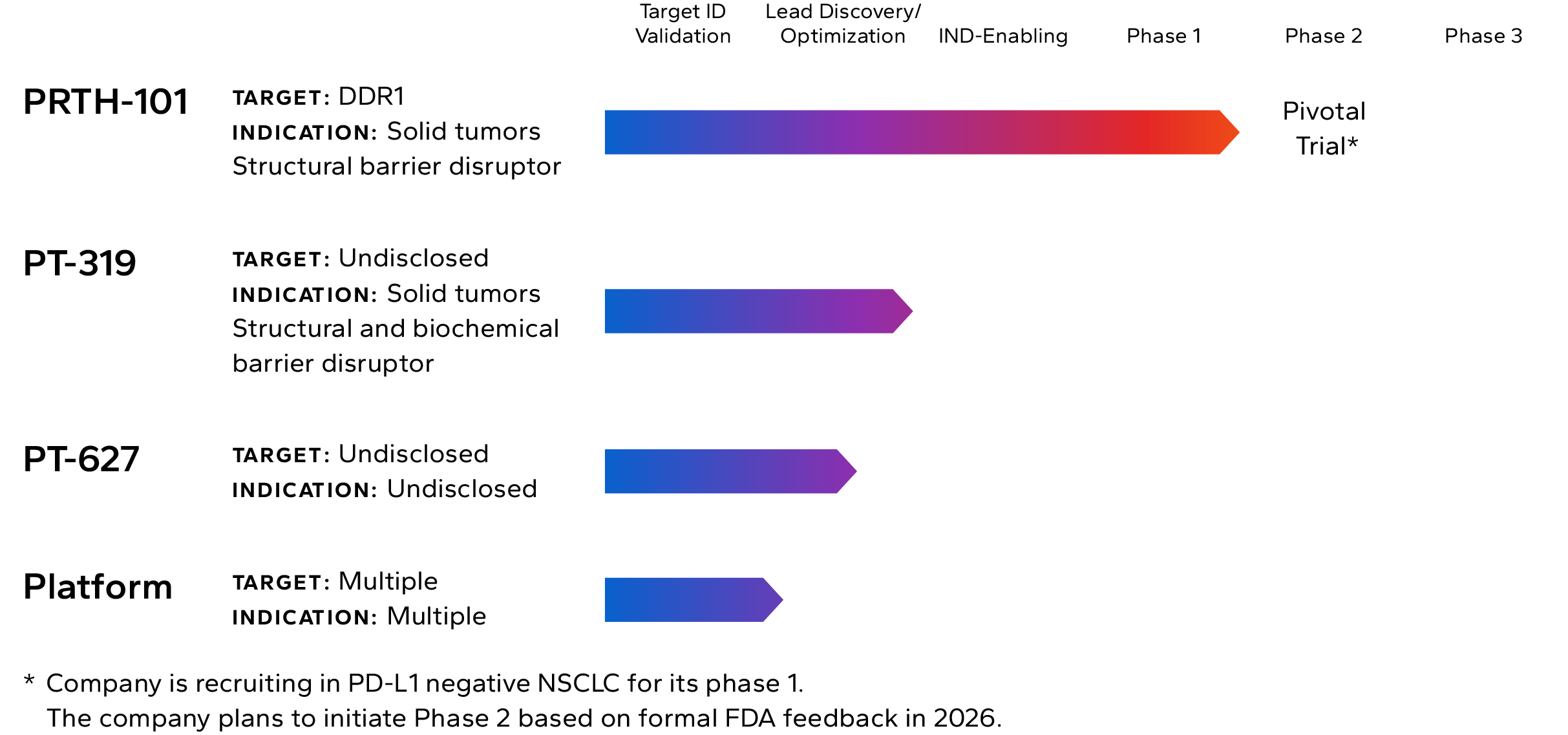
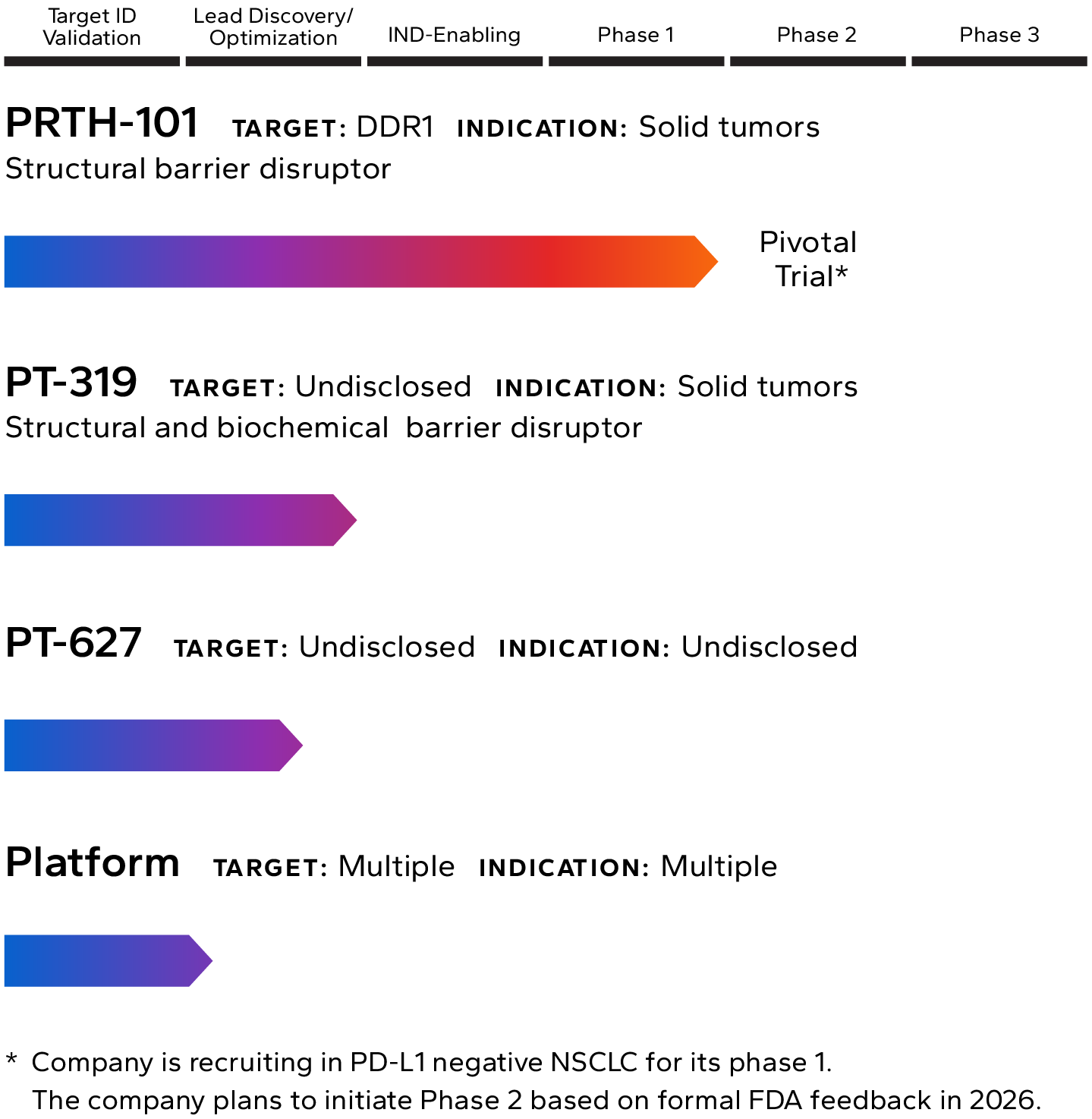
Lead Pipeline Asset
Incendia’s most advanced investigational therapeutic is PRTH-101, a novel therapeutic antibody that binds to and inhibits Discoidin Domain Receptor 1 (DDR1), an element of the TME that is highly expressed by cancer cells in multiple solid tumor types.
The Company is currently recruiting patients into a Phase 1c clinical trial with PRTH-101 alone and in combination with PD-1 inhibition for the treatment of patients with PD-L1 negative NSCLC and ICI refractory thymic carcinoma (NCT05753722). The company intends to start Phase 2 in 2026.